Heterotrophic Bacterial Population in Water, Sediment and Fish Tissues Collected From Koka Reservoir and Awash River, Ethiopia 
2. Dr. Babasaheb Ambedkar Marathwada University, Maharashtra, India.
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2015, Vol. 5, No. 11 doi: 10.5376/ija.2015.05.0011
Received: 16 Apr., 2015 Accepted: 08 May, 2015 Published: 04 Jun., 2015
Wondimu L., Sreenivasa V., Prabhadevi L., Natarajan P and Khillare Y., 2015, Heterotrophic Bacterial Population in Water, Sediment and Fish Tissues Collected From Koka Reservoir and Awash River, Ethiopia, International Journal of Aquaculture, Vol.5, No.11: 1-5 (doi: 10.5376/ija.2015.05.0011)
The quantitative estimation of total heterotrophic bacteria in the water, sediment and body tissues is helpful in predicting the quality of the fish as well as the status of the water body. The bacterial population in the water of Koka Reservoir and the Awash River, studied monthly for a period of one year, showed variation from 0.0.2×104 cfu/ml to 2.6×104 cfu/ml. Whereas, in the sediment the highest population density was 2.6 cfu/g and the lowest was 0.98×104 cfu /g. The highest population density in the reservoir was recorded in the sediment in January, while in the water was in August. The heterotrophic bacteria population in the river water and sediment was lower than the reservoir. During the non rainy season (February and March) the sediment bacteria increased with increase in water temperature and reduced rate of water level. The bacteria in the river water greatly reduced during February even though a regular pattern was not evident throughout the study period. The total bacterial population in different tissues of Cyprinus carpio and Oreochromis niloticus showed maximum population in intestine, followed by gill, skin, kidney and liver
1 Introduction
Heterotrophic bacteria are found distributed in the water as plankton as well as components of the bottom sediments in aquatic ecosystems and take part in the mineral cycling in pelagic and benthic food webs (Azam et al., 1983). Heterotrophic bacterial distribution, diversity and activities are controlled by various hydro- biological factors and nutrient levels present in the aquatic environments (Azam et al., 1983). Whipple and Rohovec (1994) reported that the temperature and pH are the limiting factors for the survival of the bacteria in the environment. Sundh and Bell (1992) found the importance of photo synthetically produced organic carbon from phytoplankton as a source of carbon for pelagic heterotrophic bacteria. (Cole et al., 1988) and Bird and Kalff (1984) observed positive relationship between phytoplankton and bacterial population in water. According to (Newton et al., 2006) the combination of continuous nutrient input and a general lack of higher trophic level food chain results in a system dominated by microbial activity. The nature of sediment and organic content plays a significant role in the proliferation and distribution of bacteria (Ogbondeminu, 1993). The bacterial production is an important parameter from public health point of view since they play a key role in water borne diseases to human as well as to aquatic organisms. In the fresh water bodies heterotrophic bacteria such as Pseudomonas sp., Aeromonas sp., Staphylococcus and E. coli are regarded as indicators of degree of pollution and sometimes responsible for diseases in cultured fishes. In culture systems several environmental parameters influence fluctuation of micro flora which in turn causes stress to fish and they become susceptible to infections by diverse groups of parasites and pathogens (Pal and Das Gupta, 1992). In order to provide a predictive capability for possible disease outbreaks and provide an opportunity to design preventative management actions, detailed information of the indicator bacterial load in the internal organs of apparently healthy fish and in the water and sediment were carried out from Koka Reservoir and the Awash River close to the reservoir.
2 Materials and Methods
Sediment and water samples were collected from Koka Reservoir and the Awash River mouth for enumeration of bacterial population from June 2010 to May 2011. The samples were collected on sterilized containers aseptically and kept in ice box in order to avoid temperature variations. Pour plate method was performed using Nutrient agar (HiMedia) after making appropriate serial dilution of the samples. The plates were incubated at 37℃. The enumeration of the bacterial colonies was done after 24 hours and recorded as number of colonies, CFU/ml for water and CFU/g for sediment sample (Garrity, 2001). The bacterial population in the tissues like skin, gills, kidney, liver and intestine of Oreochromis niloticus and Cyrpinus carpio were counted after using the tissue extracts, serially diluted and plated on nutrient agar and incubated at 35℃ after 24 hours. The bacterial population on the plates was recorded as CFU/g of tissue.
3 Results
3.1 Heterotrophic Bacterial Population in Water and Sediment
The total heterotrophic bacterial (HB) colonies in the reservoir water and in sediment are presented in Figure 1. The bacterial colonies in the reservoir water were at high levels during July, August and September. The highest HB (2.6 ×104 cfu/ml) was observed in August. The HB in the reservoir water varied from 0.65 ×104 cfu/ml to 2.6 ×104 cfu/ml. In the sediment, the bacterial count was higher than in the water in most observations. In the sediment the highest colony (2.6 ×104 cfu/ml) was recorded in January. The colony number varied between 0.98 ×104 cfu /g to 2.6×104 cfu/g during the study period.
In the River water the HB varied from 0.2 ×104 cfu/ml to 2.01 ×104 cfu/ml. However, the bacterial count in the sediment varied from 1.02 ×104 cfu /ml to 2.3 ×104 cfu/ml. In general, the bacterial count was higher in the water from June to January and in sediments during September, February and March (Figure 2).
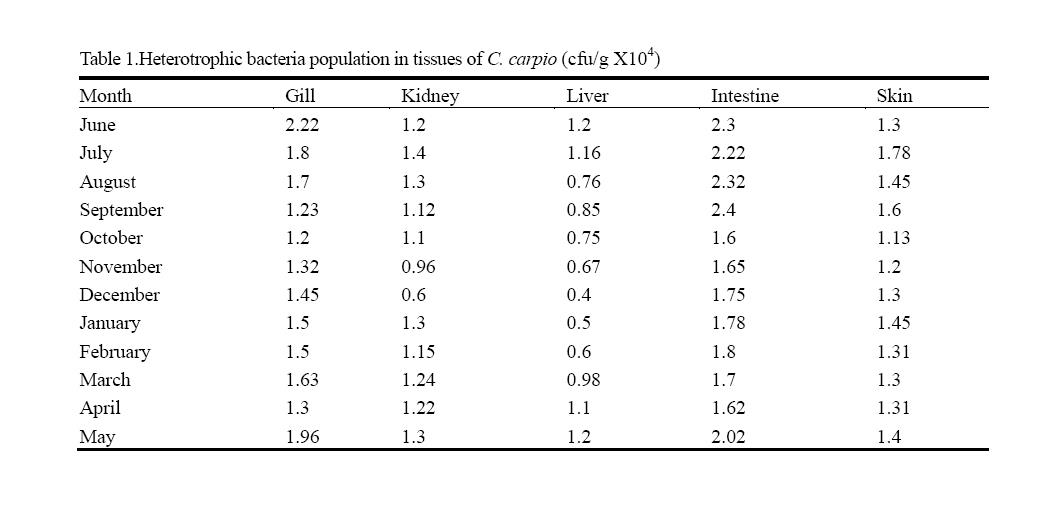
3.2 Bacteria Population in the Tissues of Cyprinus carpio
The total HB in different tissues of Cyprinus carpio is shown in Table 1 The bacterial colonies counted in the gills were very low (1.2 ×104 cfu/g) during October and highest in June (2.22 ×104 cfu/g). The HB in kidney showed not much variation during the study period. The minimal count (0.6×104 cfu/g) was found in December and maximum (1.4 ×104 cfu/g) in July. The bacterial counts recorded in the liver tissues were lower in December (0.4 ×104 cfu/g) and higher 1.2 ×104 cfu/g) in June and May. The intestinal bacterial flora was found to be higher than the other tissues and the values were found minimum in 1.62 ×104 cfu/g and maximum 2.4 ×104 cfu/g.
The variations in the bacterial count in the skin ranged from 1.13 × 104 cfu/g to 1.78 × 104 cfu/g. The lowest number of colonies was recorded in October and highest concentration in found in July.
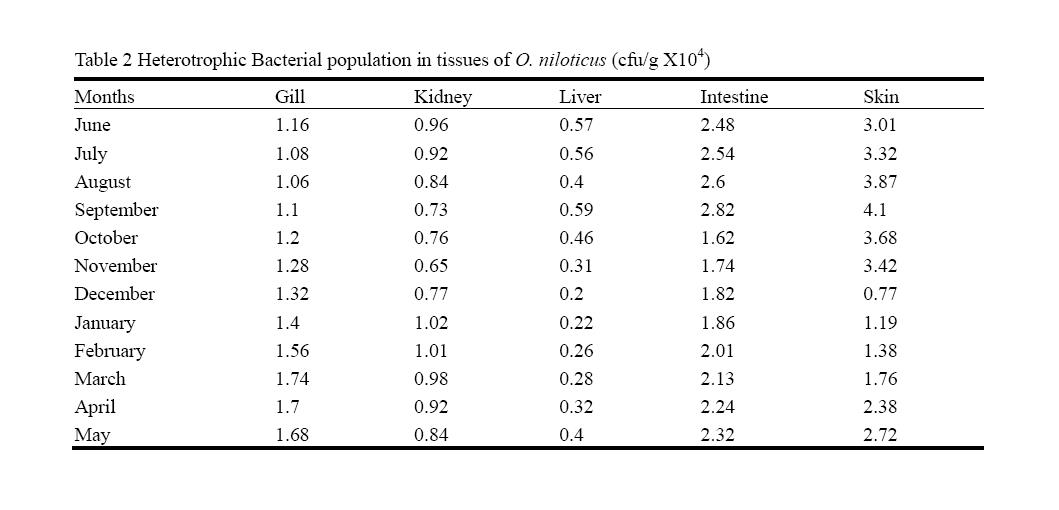
3.3 Bacterial Population in the Body Tissues of Oreochromis niloticus
The total viable bacterial counts in the gills were in the range of 1.06 x104 cfu/g to 1.74 x104 cfu/g. The HB in the kidney was found low during November (0.65 x104 cfu/g) and higher (1.02 x104 cfu/g) in January, however, the total bacterial count is comparatively lower than in the other tissues. The colony density in the liver tissues showed considerable variation (0.2 x104 cfu/g) in December 2010 and maximum (0.59 x104 cfu/g) in September. The intestinal HB varied between 1.62 x104 cfu/g and 2.82 x104 cfu/g. Generally, the population remained high from June to September and further it declined gradually. The fish skin showed comparatively higher colonies during February to May as well as June to September. The lowest was 0.77 x104 cfu/g in December and highest in September (4.1 x104 cfu/g)(Table 2).
4 Discussion
In the present study, the HB in the sediments was found to be higher when compared to water samples collected from Koka Reservoir and River sites. This might be due to the rich organic content in the sediment which acts as nutrient source. The heterotrophs utilize the organic matter and demineralize to derive the inorganic elements for their metabolism (Wollast, 1991). Anon (1997) reported that the higher bacterial colony density in the sediments than water may be due to the rich organic content and less residence time of them in water column than sediments. The bacteria in the water and sediment are closely associated with the environmental parameters such as pH, temperature and dissolved oxygen. The present results also indicated the higher density in sediments of river and reservoir during December 2010 to February 2011 after the rainy season. This may be due to stability of water column along with increase in water temperature which ultimately increases the mineralization process that facilitates the nutrients for bacterial growth. Similar observations were made by (Sugita et al., 1985) who observed the increased number of bacteria with increase in water temperature, especially summer months.
In contrast, the HB in water was found minimum in the months of February and May. This might be due to the low water levels in the reservoir and river. The present results are in conformity with that of Velammal (1993) and (Natarajan et al., 1980) who reported that during summer the low population level was maintained in water. In the present study in the month of August, highest bacterial count was noticed in the reservoir water. This result is in agreement with the observations of Martin (1981) and (Sathiyamurthy et al., 1992) who stated that during monsoon the rain water flow brings huge quantities of nutrients which promote the bacterial growth. Lokande and Shembekar (2009) have reported high number of bacterial count during monsoon season in Dhanegaon Reservoir in India.
The numerical abundance depends on the survival and proliferation of various species. In Koka Reservoir, during raining the river water discharge contains bacteria originating from the sewage and other sources. Incidence of high bacterial numbers in the water was reported from many aquatic systems (Sundari et al., 2004; Surve et al., 2006). In Koka Reservoir, bacterial counts recorded during summer months could be attributed to the extracellular products of phytoplankton (Sieburth, 1967) since there were blue-green algal blooms observed after the rainy season when the water temperature became high along with the reduction in water level.
The distribution of heterotrophic bacteria in different organs of the fish, Cyprinus carpio showed high population density during rainy season (June to September). This could be due to the introduction of bacteria from the land runoff including the sewage water that enters into the reservoir. The total bacterial counts in the various parts of the fish were studied by (Balasubramanian et al., 1992). Ogbondeminu, (1993).They reported that there is a significant correlation between bacterial population in the water and the various tissues of the fish. In general, the highest abundance of bacterial population was observed during rainy season (Ramteke and Tewari, 2002). The higher numerical density of the bacteria in the intestine, gill and skin than in the kidney and liver of both fishes indicates that the tissue mucus could act as a temporary adhering surface and serve as nourishing substance for their growth in the tissues. Similar findings were reported by Fathima (1973) and (Mary et al., 1975) in the gut of marine and estuarine fishes who stated the high bacterial population may be due to the nature of the food ingested as well as the occurrence of natural flora in the gut. Bowen (1976), Schroeder (1978) and Opuzynski (1981) stated that higher number of bacteria in the intestine of fish may be related to the detritus feeding nature of the fish. The liver and kidney showed comparatively low bacterial flora. It could be because the entry and survival of bacteria in liver and kidney are less probable compared to the mucus laden tissues. Further, the liver has detoxifying function like alkaline condition of the tissue which might prevent the entry and growth of bacteria.
References
Anon J., 1997, Ecological, toxicological and environmental assessment studies of the effluents discharge from MRLCHR in marine environs of Nagapatinam, Tamilnadu. Technology references, NIO,12/97: 86
Azam F., Fenchel T., Field J.G., Gray J.S., Meyer-Reil L.A., and Thingstad F., 1983, The ecological role of water column microbes in the sea, Mar. Ecol. Prog. Ser., 10: 257-263
http://dx.doi.org/10.3354/meps010257
Balasubramanian S., Rajon M. R., and Raj S.P., 1992, Microbiology of fish grown in sewage fed pond, Bioresource Technology, 40 (1): 63-66
http://dx.doi.org/10.1016/0960-8524(92)90120-M
Bird D.F., and Kalff J., 1984, Empirical relationships between bacterial abundance and chlorophyll concentration in fresh and marine waters,
Can. J. Fish Aquat. Sci., 41: 1015-1023
http://dx.doi.org/10.1139/f84-118
Bowen S.H., 1976, Mechanism for digestion of detrital bacteria by the cichlid fish Sarotherodon mossambicus (Peters), Nature, 260: 137-138
http://dx.doi.org/10.1038/260137a0
Cole J., Findlay S., and Pace M.L., 1988, Bacterial production in fresh and saltwater ecosystems: a cross overview, Mar. Ecol. Prog. Ser., 43:1-10
http://dx.doi.org/10.3354/meps043001
Fathima E.J., 1973, Studies on the bacterial flora of the alimentary tract of the Indian Mackeal, Dissertation submitted in partial fulfillment of Requirement for M.Sc. degree, Annamalai University, India.
Garrity G.M., 2001, Bergy’s manual of systematic bacteriology, New York, Springer Verlag.
Lokhande M.V., and Shembekar V.S., 2009, Microbilogical study of Dhanegaon Reservoir at danegaon in Osmanabad district, Maharashtra (India), Shodh, samiksha aur Mulayamkan (International Research Journal), II, 7
Martin A., 1981, Studies on Vibrio parahaemolyticus and allied vibrios from pitchavaram mangrove-killai backwater complex interconnecting the Vellar and Coleoon estuarine system, Porto-Novo, South India, PhD Thesis, Annamalai University, India.
Mary P.P., Chandramohan D., and Natarajan R., 1975, The gut microflora of some commercially important fishes from Porto Novo Waters, Bull. Dept. Mar. Sci. Univ, Cochin, VII: 185-199
Natarajan R., Abraham M., and Nair G.B., 1980, Distribution of Vibrio parahaemolyticus in Porto Novo environment, Indian J. Med. Res., 71: 679-687
Newton R.J., Kent, A.D, Triplett E.W and Katherine D. McMahon, K.D., 2006, Microbial community dynamics in a humic lake. Differential persistence of common freshwater phylotypes, Environmental Microbiology, 8 (6): 956-970
http://dx.doi.org/10.1111/j.1462-2920.2005.00979.x
Ogbondeminu F.S., 1993, The occurrence and distribution of enteric bacteria in fish and water of tropical ponds in Nigeria. J. Aquac. Trop., 8: 61-66
Opuzynski K., 1981, The influence of the silver carp, Hypophthalmichthys molitrix (Val) on eutrophication of the environment of carp ponds, Part VII: Recapitulation,. Rocz.Nauk.Roln.Ser., 94: 127-151
Pal D., and Das Gupta C.A.F., 1992, Microbial pollution in water and its effect on fish. J.Aquat.Anim.health 4, pp.329
http://dx.doi.org/10.1577/1548-8667(1992)004<0032:MPIWAI>2.3.CO;2
Ramteke P.W., and Tewari S., 2002, Comparative study of fluorogenic and chromogenic media for specific detection of environmental isolates of thermotolerant Escherichia coli, Environ. Monitoring Assess, 79: 121-127
http://dx.doi.org/10.1023/A:1020278114463
Sathiyamurthy K., Purushothaman A., and Ramaiyan V., 1992, Heavy metal and drug resistant bacteria in the Vellar estuary, South East Coast of India, Mahasagar, 25: 119-122
Schroeder G. L., 1978, Autotrophic and heterotrophic production of microorganisms in intensively manured fish ponds and related fish yields, Aquaculture, 14(4): 303-325
http://dx.doi.org/10.1016/0044-8486(78)90014-5
Seiburth J.Mc.N., 1967, Seasonal selection of estuary bacteria by water temperature, J.Exp.Mar.Biol. Ecol., 1: 98-121
http://dx.doi.org/10.1016/0022-0981(67)90009-3
Sugita H., Fushino T., Oshima K., and Deguchi Y., 1985, Microflora in the water and sediment of fresh water culture ponds. Bull.Jap.Soc.Sci.Fish., NISSUISHI, 51: 91-97
Sundari V.,Valliappan R., and Shelvamani A., 2004, Bacteriological quantity of drinking water in and around Chidambaram Taluka of Cuddalur district, Poll.Res., 23(4): 757-759
Sundh I., and Bell R.T., 1992, Extracellular organic carbon released from phytoplankton as a source of carbon for heterotrophic bacteria in lakes of different humic content. Hydrobiologia, 229: 93-106
http://dx.doi.org/10.1007/BF00006993
Surve P.R., Amber N.E., and Pulle J.S., 2006, Microbiological study of two fresh water bodies Barul and Kamdar dam in Nanded district, India, Poll.Res., 25(1): 95-96
Velammal A., 1993, Studies on Vibrio Cholerae and Vibrio Parahaemolyticus from Pondicherry Coastal Environs South India. PhD Thesis, Annamalai University, India.
Whipple M.J., and Rohovec J.S., 1994, The effect of heat and low pH on selected viral and bacterial fish pathogens, Aquaculture, 123:179-189
http://dx.doi.org/10.1016/0044-8486(94)90056-6
Wollast R., 1991, The coastal carbon cycle: fluxes, sources and sinks, In: Mantoura R.F.C., Martin J.M., and Wollast R. (eds.), Ocean margin processes in Global change, J.Wiley and Sons, Chichester, pp.365-382
. PDF(365KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Lakew Wondimu
. Sreenivasa V
. Prabhadevi L
. Natarajan P
. Khillare Y
Related articles
. Cyprinus carpio
. Oreochromis niloticus
. Heterotrophic Bacteria
. Body Tissues
Tools
. Email to a friend
. Post a comment